2022年12月の新規レジメン申請用資料
今月は新規の3つのレジメンの申請申請しています。デュルバルマブはイミフィンジ、トレメリムマブはイジュドの商品名で承認されています。
非小細胞肺癌(一次治療)
デュルバルマブ+トレメリムマブ+化学療法 併用療法

PURPOSE The open-label, phase III POSEIDON study evaluated tremelimumab plus durvalumab and chemotherapy (T + D + CT) and durvalumab plus chemotherapy (D + CT) versus chemotherapy alone (CT) in first-line metastatic non–small-cell lung cancer (mNSCLC). METHODS Patients (n = 1,013) with EGFR/ALK wild-type mNSCLC were randomly assigned (1:1:1) to tremelimumab 75 mg plus durvalumab 1,500 mg and platinum-based chemotherapy for up to four 21-day cycles, followed by durvalumab once every 4 weeks until progression and one additional tremelimumab dose; durvalumab plus chemotherapy for up to four 21-day cycles, followed by durvalumab once every 4 weeks until progression; or chemotherapy for up to six 21-day cycles (with or without maintenance pemetrexed; all arms). Primary end points were progression-free survival (PFS) and overall survival (OS) for D + CT versus CT. Key alpha-controlled secondary end points were PFS and OS for T + D + CT versus CT. RESULTS PFS was significantly improved with D + CT versus CT (hazard ratio [HR], 0.74; 95% CI, 0.62 to 0.89; P = .0009; median, 5.5 v 4.8 months); a trend for improved OS did not reach statistical significance (HR, 0.86; 95% CI, 0.72 to 1.02; P = .0758; median, 13.3 v 11.7 months; 24-month OS, 29.6% v 22.1%). PFS (HR, 0.72; 95% CI, 0.60 to 0.86; P = .0003; median, 6.2 v 4.8 months) and OS (HR, 0.77; 95% CI, 0.65 to 0.92; P = .0030; median, 14.0 v 11.7 months; 24-month OS, 32.9% v 22.1%) were significantly improved with T + D + CT versus CT. Treatment-related adverse events were maximum grade 3/4 in 51.8%, 44.6%, and 44.4% of patients receiving T + D + CT, D + CT, and CT, respectively; 15.5%, 14.1%, and 9.9%, respectively, discontinued treatment because of treatment-related adverse events. CONCLUSION D + CT significantly improved PFS versus CT. A limited course of tremelimumab added to durvalumab and chemotherapy significantly improved OS and PFS versus CT, without meaningful additional tolerability burden, representing a potential new option in first-line mNSCLC.
https://ascopubs.org/doi/full/10.1200/JCO.22.00975
第3相POSEIDON試験(JCO 2022)
肝細胞癌(一次治療)
デュルバルマブ+トレメリムマブ 併用療法
https://evidence.nejm.org/doi/full/10.1056/EVIDoa2100070
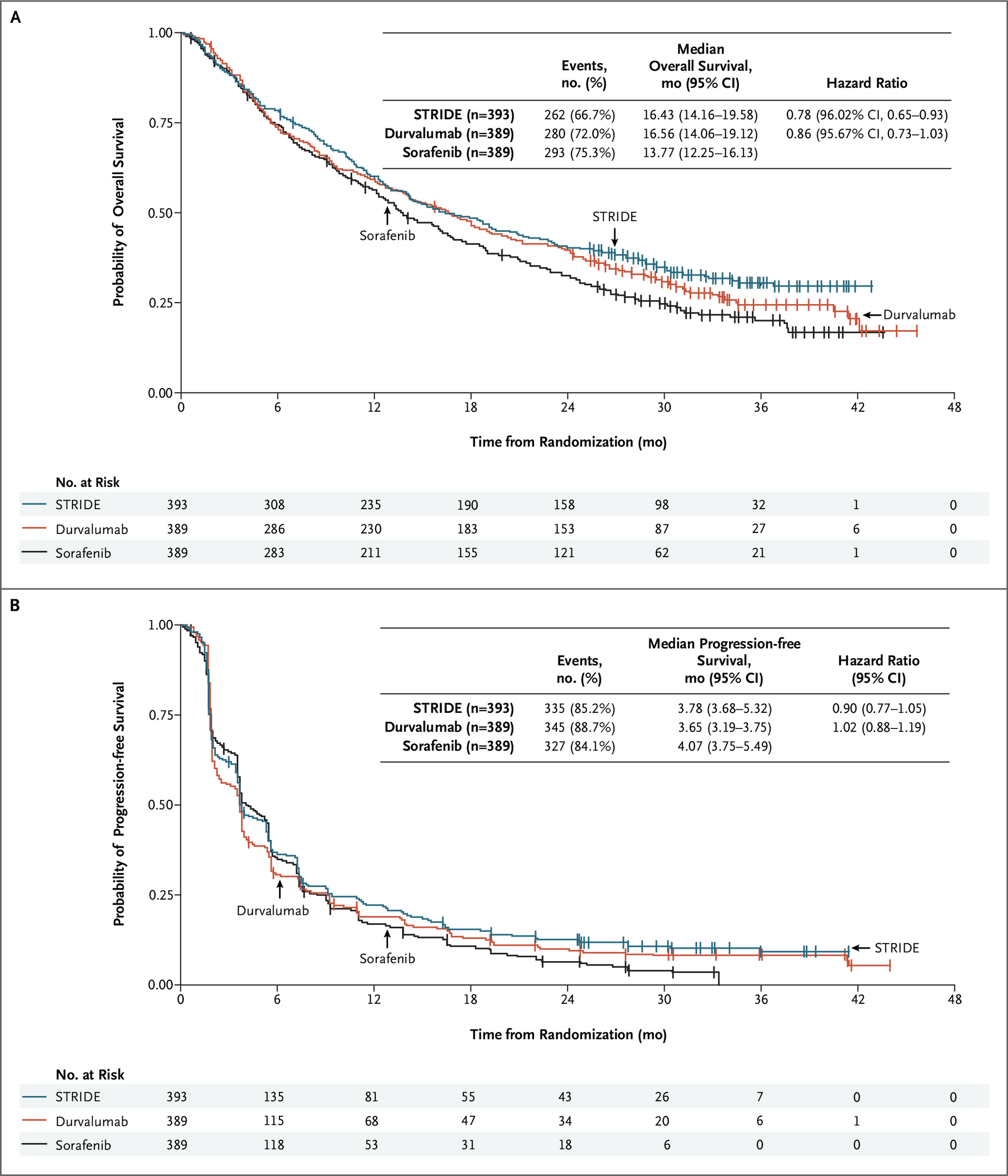
第2相HIMALAYA試験(NEJM Evid. 2022 Jun 6.)
PD-L1抗体デュルバルマブとCTLA-4抗体トレメリムマブの併用療法のSTRIDEレジメンをデュルバルマブ単剤およびソラフェニブと比較し、OSが16.43ヶ月 vs 16.56ヶ月 vs 13.77ヶ月でソラフェニブに対して有意な延長を示した。デュルバルマブ単独療法はソラフェニブに対する非劣性を示した。
肝内胆管癌(一次治療)
デュルバルマブ+GEM+CDDP 併用療法
https://evidence.nejm.org/doi/full/10.1056/EVIDoa2200015
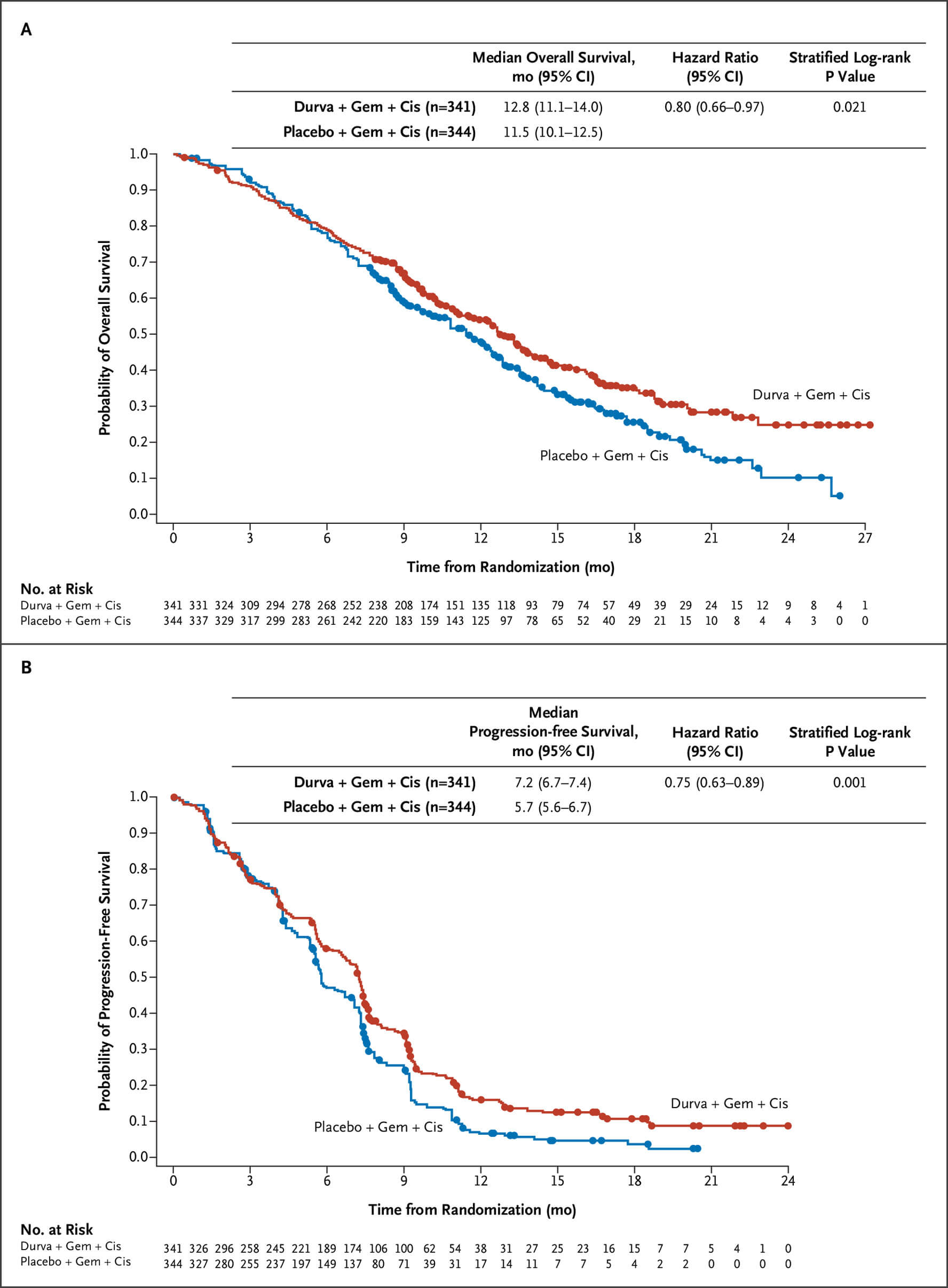
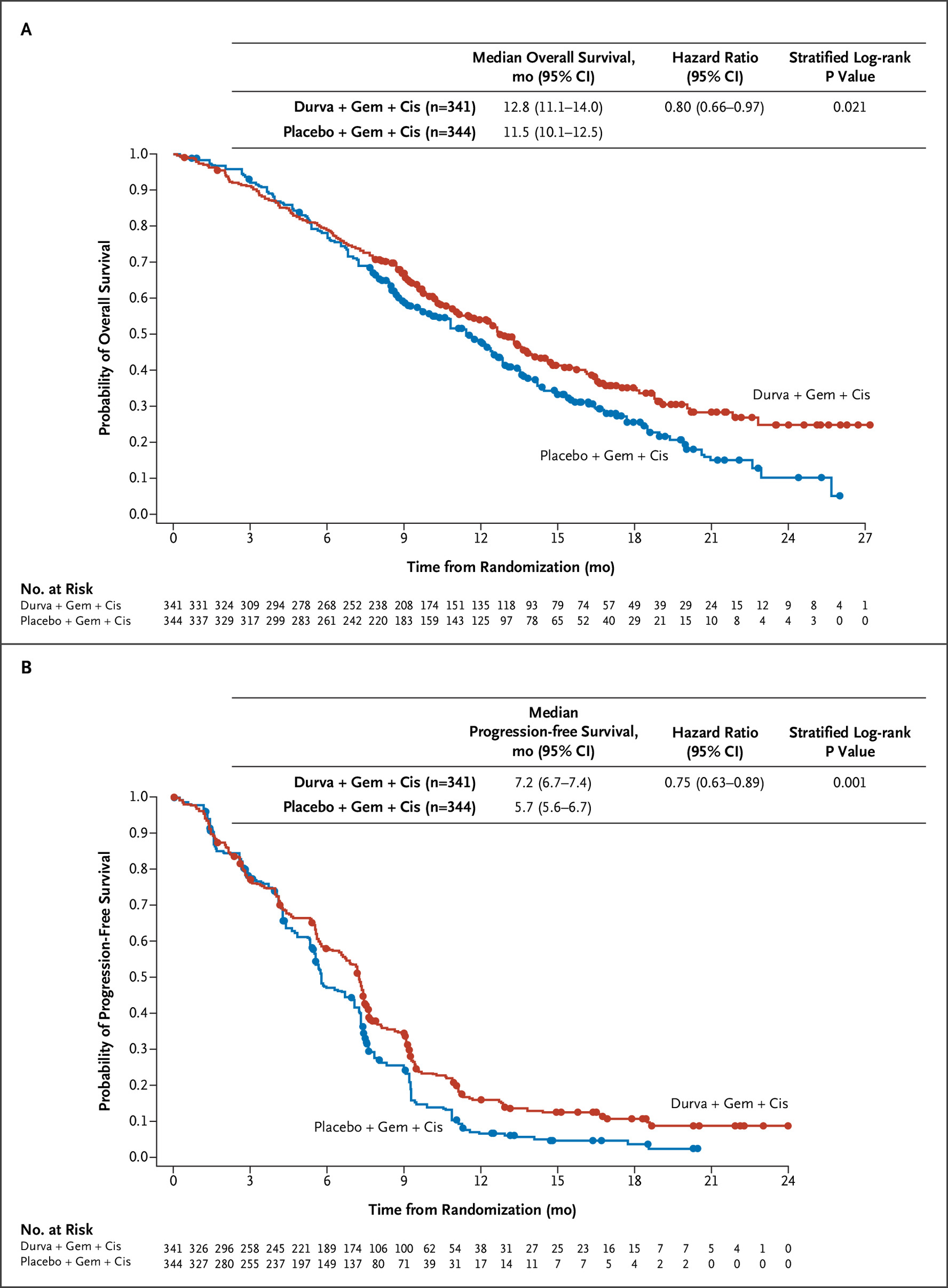
第3相TOPAZ-1試験(NEJM Evid. 2022)
GEM+CDDPにデュルバルマブを上乗せすることで、2年生存率が10.4→24.9%に改善。OS HRは0.80(95%CI 0.66-0.97)、PFS HRは0.75(95%CI 0.63-0.89)。
この記事に対するコメント
このページには、まだコメントはありません。
更新日:2022-12-25 閲覧数:885 views.